| Strontium | |
|---|---|
 | |
| Identification | |
Symbol |
Sr
|
Block |
s-Bloack
|
Group |
Group 2
|
Period |
Period 5
|
| Atomic Information | |
Atomic Number |
38
|
Atomic Radius |
215 pm
|
Mass |
87.62
|
Category |
Akaline earth Metals
|
Standard state(298 K) |
Solid
|
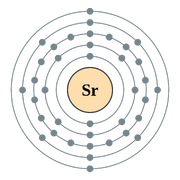
Electronic Configuration |
[Kr]5s2
|
Electronegativity (Pauling) |
0.95
|
Unknown
| |
First ionisation energy |
549.5 kJ/mol
|
| Physical Properties | |
Color |
Unknown
|
Melting Point |
1050 K
|
Boiling Point |
1650 K
|
Density of solid |
2.64 g/cm3
|
Unknown
| |
| Heat Properties | |
Enthalpy of fusion |
7.43 kJ/mol
|
Enthalpy of atomisation |
Unknown
|
Enthalpy of vaporisation |
141 kJ/mol
|
| 4 Be |
| 12 Mg |
| 20 Ca |
| 38 Sr |
| 56 Ba |
| 88 Ra |
Strontium is the 38th element in the periodic table. It is a soft, silvery metal that burns in air and reacts with water. Strontium is best known for the brilliant reds its salts give to fireworks and flares. It is also used in producing ferrite magnets and refining zinc.
Modern ‘glow-in-the-dark’ paints and plastics contain strontium aluminate. They absorb light during the day and release it slowly for hours afterwards.
Strontium-90, a radioactive isotope, is a by-product of nuclear reactors and present in nuclear fallout. It has a half-life of 28 years. It is absorbed by bone tissue instead of calcium and can destroy bone marrow and cause cancer. However, it is also useful as it is one of the best high-energy beta-emitters known. It can be used to generate electricity for space vehicles, remote weather stations and navigation buoys. It can also be used for thickness gauges and to remove static charges from machinery handling paper or plastic.
Strontium chloride hexahydrate is an ingredient in toothpaste for sensitive teeth.
History[]
In 1787, an unusual rock which had been found in a lead mine at Strontian, Scotland, was investigated by Adair Crawford, an Edinburgh doctor. He realised it was a new mineral containing an unknown ‘earth’ which he named strontia. In 1791, another Edinburgh man, Thomas Charles Hope, made a fuller investigation of it and proved it was a new element. He also noted that it caused the flame of a candle to burn red.
Meanwhile Martin Heinrich Klaproth in Germany was working with the same mineral and he produced both strontium oxide and strontium hydroxide.
Strontium metal itself was isolated in 1808 at the Royal Institution in London by Humphry Davy by means of electrolysis, using the method with which he had already isolated sodium and potassium.
| 1 H |
2 He | ||||||||||||||||
| 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||
| 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||
| 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
| 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| 55 Cs |
56 Ba |
* | 72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 87 Fr |
88 Ra |
** | 104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og |
| 119 Uue |
120 Ubn |
*** | 158 Ups |
159 Upo |
160 Upe |
161 Uhn |
| * | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
| ** | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
